Shockwave generators for medical use manufactured by SANUWAVE Inc. adopt PACE® (Pulsed Acoustic Cellular Expression) technology which activates biological signals and angiogenic reactions, leading to new vascularization and improved microcirculation, thus helping the body to restore normal healing processes.

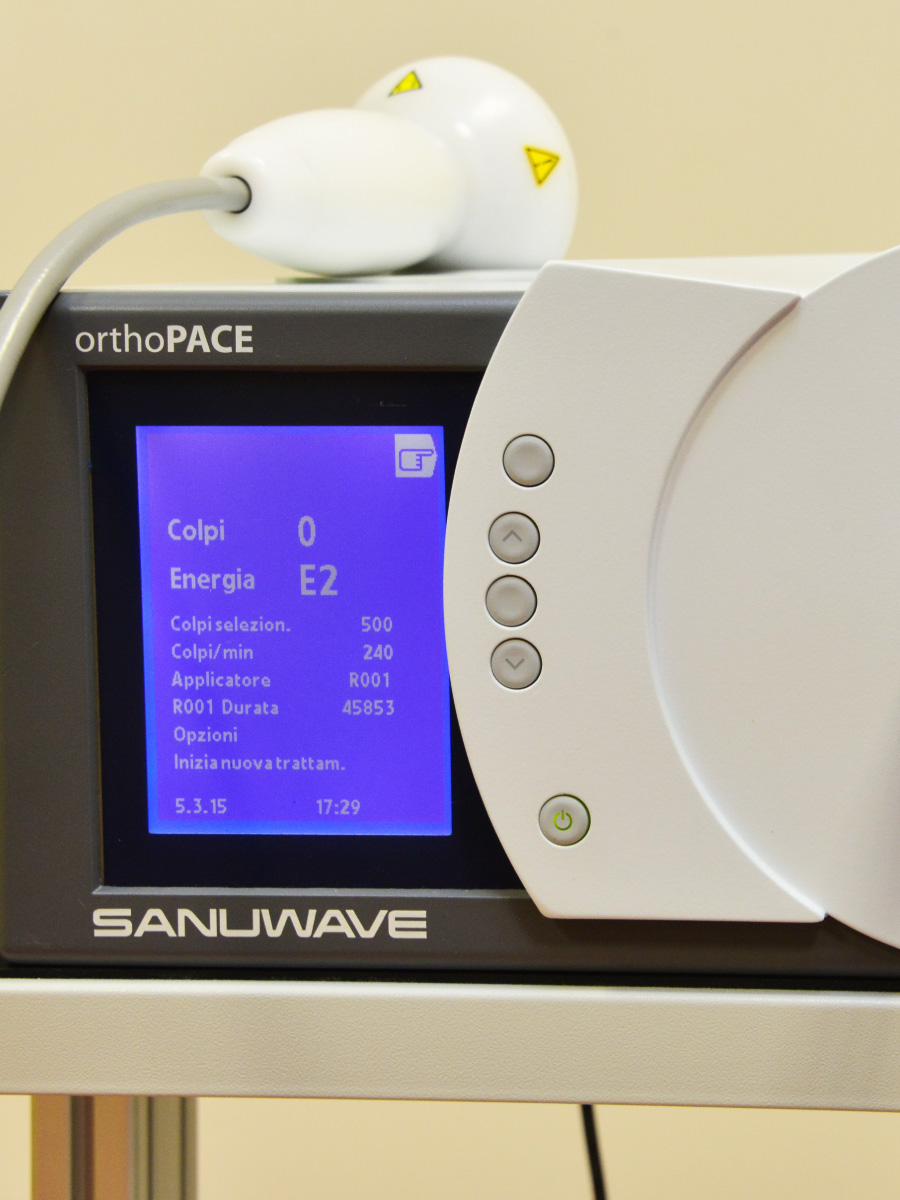
orthoPACE®
Cutting-edge technology
It generates powerful electro-hydraulic shock waves characterized by an ultra-fast ascending phase and a very high pressure peak. An extended focal area ensures consistent energy distribution throughout the area to be treated, through applicators available in different sizes based on the required depth. Also available sterile protective drapes and sterile gel.
Easy to use
The ergonomically shaped applicator is connected to the central unit with a flexible cable that facilitates its positioning; the plug-and-play technology and the intuitive interface make treatment easy and efficient and the compact design allows it to adapt to confined environments in limited spaces.
Affordable cost
Two or three applications are enough for a satisfactory therapeutic result; the payback time is short even with a few patients per month and each single treatment lasts a few minutes.
For a wide range of applications
- insertional tendinopathy
- tendinitis
- calcific tendinosis of the shoulder
- rotator cuff syndrome
- epicondylitis (tennis elbow, golfer's elbow)
- plantar fasciitis
- subcalcaneal and retrocalcaneal spur
- achillodynia
- calcific insertional tendinopathy of the Achilles
- patellar apex syndrome (insertional tendinopathy of the patellar)
- trochanteric bursitis
- delayed union and pseudarthrosis after fracture
- groin pain
- induratio penis Plastica
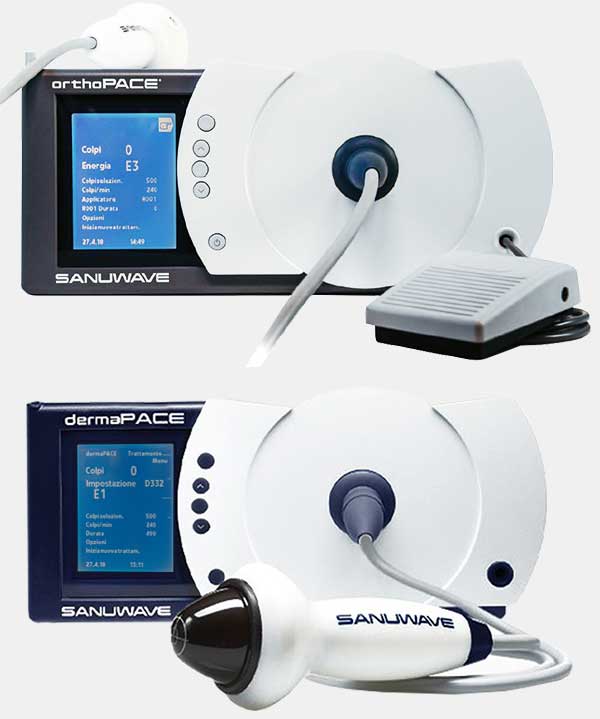
dermaPACE®
dermaPACE® revolutionizes the approach to wound care with the aim of promoting the healing process of even the most difficult cases to treat. Thanks to the patented technology that exploits acoustic energy in pulsed mode, dermaPACE® promotes cellular expression (PACE®) and provides an effective solution for the treatment of acute and chronic wounds and injuries and subcutaneous soft tissues.
In 2018, the device obtained certification from the FDA (Food and Drug Administration - American Agency for Food and Medicines, which aims to protect the health of citizens) for the treatment of chronic conditions and for the treatment of ulcers of the diabetic foot that does not exceed 16 cm2 and that extends through the epidermis, dermis and tendons (cannot be used in the presence of exposed bones). This system for external use was specifically designed for adult patients aged 22 or over who have had diabetic foot ulcers for more than 30 days.
Effectiveness
It starts the wound healing process (reduction of the healing time) and improves the patient's quality of life: pain reduction, comfort and safety with rare side effects and few contraindications.
Easy to use
Kit preset parameters (compact)
Quick treatment: ~ 2 minutes (for wounds up to 64 cm2)
It requires few treatments
No risk of allergic reactions
Adaptable to standard for wound care protocol
Only one therapeutic head available with a depth of penetration up to 9 mm
Affordable cost
Faster wound healing process
Fewer dressings
Modest investment
Lower costs for checks
For a wide range of applications
- Diabetic ulcers
- Ischemic ulcers
- Venous insufficiency ulcers
- Mixed ulcers
- Pressure ulcers
- 2nd degree burns
- Cloudy post-traumatic wounds
- Post-surgical or post-traumatic skin healing delays
- Cellulite
- Stretch marks
- Scars from trauma wounds, from surgical incisions, from liposuction
